For several years, I was part of a project developing energy systems built around massively powerful alternator-type devices; the one I have now will generate up to 8 kilowatts (kW). To put this in perspective, that would be 666 amps at 12 volts! (See the Integrel system at OceanPlanetEnergy.com; in full disclosure, please note that I now have a commercial interest in OceanPlanet and the Integrel system.) But, there is not a lot of point in having this kind of generating capability if there is nowhere to dump and store the energy.
The “holy grail,” from my marine energy perspective, has always been a system capable of generating and storing — during normal propulsion engine run times — sufficient energy to run the house systems for 24 or more hours. In our case, normal engine run time is the time it takes to set or pull up the anchor, or to get on and off a dock. For those with air conditioning, the system would need to be able to replenish the energy used overnight during normal boat operations (i.e., without requiring a stand-alone generator).
For this kind of system, a battery is needed that can store substantial amounts of energy in a relatively compact and lightweight format (a high energy density), and is capable of absorbing extremely high charge rates to high states of charge. It must be able to tolerate deep discharges so that the full capacity can be used at each cycle. This is the battery a typical cruising sailor needs, albeit on steroids in our application. There are various lithium-ion offerings that meet these requirements, but in general these are shockingly expensive (on paper, up to 10 times as expensive for the same nominal capacity as lead-acid; although, as we will see shortly, this is not a fair comparison) so let’s first take a look at lead-acid.
 |
|
High-output alternators can get really hot. |
Lead-acid limitations
All forms of lead-acid batteries have major drawbacks in a cruising environment. First, they don’t like deep discharges. In order to minimize the depth of discharge at each cycle, you need at least double the capacity you will use, which translates into a lot of volume, weight and cost. When it comes time to recharge, once you get up to around 50 to 60 percent state of charge, the batteries’ ability to absorb charging current steadily tapers off and can’t be forced without doing damage. If the operator gets fed up with the extended engine or generator run time resulting from the low charge acceptance rate and repeatedly terminates the charge before the batteries are fully charged (this is known as operating in a partial state of charge), the batteries suffer damage from sulfation.
Lead-acid batteries are relatively inefficient at converting electrical energy into chemical energy and vice versa. This inefficiency is manifested as heat. If you force the pace on the charging side, a lot of heat is generated (I have scorch marks in the bottom of my battery compartment!). If the battery gets too hot, it goes into a condition known as thermal runaway, in which the internal electrolyte boils, producing hydrogen and oxygen. In the worst cases, the battery blows up, or the vented hydrogen accumulates inside the boat, is ignited by a spark, and the boat blows up.
AGM rules
Of all the lead-acid batteries currently on the market, the absorbed glass mat (AGM) family has the best overall properties for my operating environment and that of most cruising boats. In particular, these batteries have the highest charge acceptance rate of any lead-acid battery, to the highest states of charge, and the highest efficiency (around 85 percent, as opposed to around 60 percent for wet-cells). As such, they also generate the least heat during fast charges and discharges.
 |
|
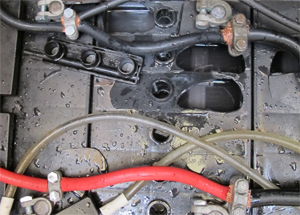
Hydrogen explosions from venting lead-acid batteries that have been pushed too hard with charging current. |
Within the conventional AGM family, we have a subgenre described as thin plate pure lead (TPPL), the best known of which are the Odyssey and PowerSafe SBS batteries from EnerSys, and the NSB Energy1 batteries from Northstar. These batteries will tolerate repeated deep discharges, down to around 20 percent remaining capacity. Over the past decade, I have participated in aggressive testing of dozens of these batteries. In general, they have performed well, except that unfortunately they do not like being operated in a partial state of charge, suffering from sulfation. This means you regularly (ideally, once a week) have to have an extended charge cycle at low rates of charge.
If you have to run an engine (the main engine or a generator) solely for the full charge cycle, it is extraordinarily inefficient. An antidote to this is to have sufficient solar on board, but it must be configured so that it is providing the necessary extended charge cycle (as opposed to being consumed by house loads). An even better antidote would be to eliminate the sulfation issue and thus the need for the extended full charge cycle.
The magic of carbon
Some years ago, it was discovered that if you sprinkle carbon dust into the active material in the negative plates of an AGM battery, the carbon inhibits sulfation. There is a family of these batteries available from Northstar known as the NSB Blue batteries. I have yet to test any, but this looks to be a significant step forward for partial state of charge operation.
The ultimate battery in the carbon-doping world comes from Firefly. In a “normal” lead-acid battery, there are plates with a grid composed of lead (this conducts the energy in and out of the battery) into which is pasted the active material (this absorbs energy on charging and gives it up on discharging). A Firefly battery dispenses with the lead grid in the negative battery plate and replaces it with a foam formed from carbon. The active material is pasted into the pores in the foam. The carbon acts as the conductor of energy in and out of the battery plate.
 |
|
A burned shore-power connection from hybrid experiments aboard Nada. |
This foam results in some remarkable changes in battery characteristics. The normal consequence of discharging a battery is to turn the active material into lead sulfate. If the battery is left in a discharged state, the lead sulfate slowly morphs into large crystals that cannot be recovered by normal charging processes (this is the sulfation mentioned above). However, the pore structure in the Firefly foam is too small to allow the lead sulfate to crystallize to this extent, and as a result these batteries are more or less immune to sulfation. Does this sound too good to be true? I have operated these batteries in a partial state of charge for months at a time, and I have discharged them to 35 percent state of charge and left them for eight months without recharging them. I have then restored them to 100 percent state of charge.
The carbon-foam plate grid is a major step forward in the lead-acid battery world and unquestionably the best for partial state of charge operation. The technology is patented and, as such, these batteries are only available from Firefly.
From lead-acid to lithium-ion
After more than a century of development, you’d think we’d know everything there is to be known about lead-acid batteries, but exciting discoveries are still being regularly made; lead-acid technology should not be written off. However, there is nothing on the horizon that suggests lead-acid batteries will absorb the kind of charging currents I can now throw at them up to high states of charge, and at efficiency levels that will limit the internal heat generation in the battery. For this, the only game in town is lithium-ion.
Lithium-ion batteries have several times the energy storage capacity of lead-acid batteries of an equivalent volume and weight. They can be charged at extraordinarily high rates of charge to very high states of charge, discharged almost totally without damage anywhere from hundreds of times to thousands of times, and they are immune to sulfation and therefore can be operated permanently in a partial state of charge (and, in fact, they prefer it). Whereas the most efficient lead-acid batteries are 85 percent efficient at converting electrical energy into chemical energy and vice versa, lithium-ion is better than 95 percent efficient, resulting in far less heat generation during high-rate discharges and recharges. This is an amazing set of positive characteristics. There are, however, some potential negatives.
 |
|
Nigel capacity testing various batteries in his garage. |
Preventing fires
Every lithium-ion battery currently in the marine marketplace contains a flammable electrolyte. All lithium-ion batteries can be driven into an exothermic state — one in which the battery generates heat internally, even when disconnected from charging sources and loads. Once initiated, this exothermic reaction can be hard to stop, resulting in a rapid temperature rise (thermal runaway) that aggravates the situation. Depending on the chemistry, the battery may get hot enough to set itself on fire (as happened on multiple Boeing 787s in 2013); but, even if the chemistry cannot get this hot, there will be a pressure rise that frequently leads to venting of the electrolyte. If there is any kind of an ignition source, the electrolyte will catch fire. Once the electrolyte lights up, it generally cannot be put out — the normal result is the loss of the boat (and there have already been a number of losses due to this exact scenario).
Conditions that can initiate thermal runaway include overcharging, over-discharging followed by a recharge, charging in freezing temperatures, operating in high ambient temperatures, manufacturing defects, external heat and physical damage. Several of these conditions are not uncommon in marine applications! What is more, whereas in automotive and other mass applications the battery builder and installer have near complete control over the installation for its entire life, once a boat gets into the hands of its owner there is no telling who will mess with what installation and wiring over the life of the boat.
To ensure the safety of lithium-ion batteries in the boating world, a sophisticated battery management system (BMS) is required that, at a minimum, monitors voltage and temperature at the individual battery cell level, and has mechanisms to shut the battery down if it is abused or if any one cell begins to drift outside designated parameters. An effective BMS is expensive to develop and implement. Without it, the battery can threaten the health of the boat.
 |
|
Nigel chopping up batteries to determine failure modes. |
Drop-in replacement?
There are lithium-ion battery manufacturers advertising their products as “drop-in” replacements for lead-acid batteries. It would be foolish to interpret this too literally. Fundamentally, an energy system built around lithium-ion batteries needs to be a fully engineered system in which the battery BMS is integrated with — and has a degree of control over — all the charging devices on the boat. In the absence of this, the default safety mechanism for the battery’s BMS is to disconnect the battery from the boat if the BMS sees a condition that threatens the health of the battery. But if this disconnection takes place without first shutting down charging devices, there can be a voltage spike through the boat that blows out sensitive electronics. In other words, the battery protects itself at the expense of the boat.
A Yanmar Technical Bulletin from June 2017 addresses this issue as follows: “Always keep a lead-acid battery in the system directly connected to the alternator. This to supply the engine ECU and starter motor and prevent spikes in the system when the BMS stops the charging of the Li-ion battery.”
Chemistry choices
Two chemistries predominate in the marine lithium-ion world: lithium iron phosphate (LFP), and nickel manganese cobalt (NMC). If LFP is driven into thermal runaway, it will not generate high enough temperatures to set itself on fire, whereas NMC can. For this reason, LFP has often been described as intrinsically safe and has been promoted as the only suitable chemistry for marine applications. However, as noted above, the electrolyte is still flammable and there have been some notable fires and boat losses.
Within the LFP and NMC families, there are literally dozens of variations in terms of construction, chemical doping, protective measures, etc. Given the correct BMS and packaging, it is arguable that NMC can be made as safe as LFP; there are certainly some NMC batteries that are safer than some LFP batteries. Regardless of chemistry, the only lithium-ion batteries that I will consider putting in my boat must have been subjected to rigorous third-party abuse testing to a recognized standard (notably Underwriters Laboratories UL 1973) and/or come from a respected marine brand with a significant lithium-ion track record.
 |
|
Torqeedo lithium-ion batteries installed on Nada. |
Cost comparisons
Once the safety issues are resolved, cost becomes the major drawback of lithium-ion batteries. The individual cells may not be that expensive, but they have to be packaged into a battery and include an effective BMS. This BMS needs to be custom developed for the relatively low-volume marine marketplace. The net result is that it is rare to find a marine lithium-ion battery that retails for less than $1,000 per kilowatt-hour (kWh) of capacity, and some run as high as $2,000. By comparison, a 100 amp-hour (Ah), 12-volt, lead-acid battery has a nominal capacity of 1.2 kWh with a cost of one-tenth to one-fifth that of a lithium-ion battery with the same capacity.
Happily for lithium-ion, this cost comparison is grossly misleading! At best, the lead-acid battery will only deliver half its capacity each time it is discharged and recharged (cycled), whereas lithium-ion will easily deliver 70 percent of its capacity. The lead-acid battery can only be cycled hundreds of times before it fails, whereas lithium-ion — depending on its chemistry and construction — may be cycled thousands of times. A true measure of the cost of a battery is how much energy can be cycled through it during its lifetime (what I like to call the lifetime kilowatt-hour throughput), divided by the battery’s purchase price. This will yield a cost per kWh of throughput. In many applications, if the capabilities and cycle life of lithium-ion can be fully exploited (in practice, this is often not the case), even at today’s prices lithium-ion will have a lower cost per kWh of throughput than any lead-acid battery.
The deeper we delve, the better it gets for lithium-ion. Let’s say I am running my main engine or generator to battery charge at anchor. We have to include the engine run time, fuel and maintenance in the cost of the energy being produced, along with the battery cost per kWh of throughput. If I have an 8-kW charging device and I have lithium-ion batteries that can absorb the full 8 kW to a high state of charge, whereas lead-acid batteries will (on average) only absorb half this, then I can cut the engine run time for battery charging in half. If I run the numbers, I will find this dramatically reduces my cost of energy, more or less regardless of the cost of the lithium-ion batteries.
 |
|
A large thin plate pure lead (TPPL) battery bank on Nada for hybrid power experiments. |
Both LFP and NMC have the properties we need for my 8-kW alternator-type device. A relatively small battery pack, rated at around 10-kWh capacity, is able to absorb the full output of the machine up to almost a 100 percent state of charge. The over 95 percent battery efficiency ensures that little charging energy is wasted as heat. The ability to withstand near 100 percent discharges for thousands of cycles enables us to utilize at least 7 kWh of the 10-kWh battery capacity at each cycle. The immunity to damage from sulfation permits operation in a partial state of charge whenever, and for however long, we want.
With lithium-ion applications in mind, we designed our alternator-type device such that, even if it is open-circuited at full output, it will not generate a damaging spike: If the battery BMS disconnects the batteries, the boat’s electronics will not blow out. There is no need to have a lead-acid buffer battery in the system.
A step change in DC systems
We are entering a new era in terms of energy systems on boats, with extraordinarily high-rate charging devices and batteries that can absorb pretty much anything we can throw at them.
On our boat, with our current energy needs of less than 3 kWh a day, one battery charge keeps us going for up to three days. Or, 20 minutes spent setting or retrieving the anchor, or getting in and out of a slip, gives us the energy we need for 24 hours. We’ve had this setup for three years now, but I’m still blown away by it! It’s the energy system I’ve always dreamed of.
Nigel Calder is a member of the ABYC Electrical Project Technical Committee, and author of numerous books including Boatowner’s Mechanical and Electrical Manual.

